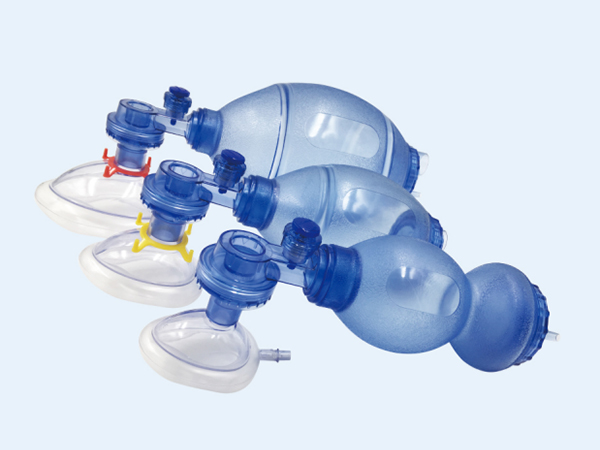
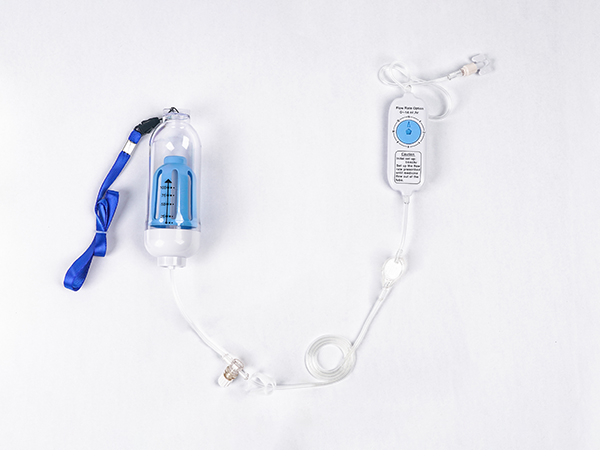
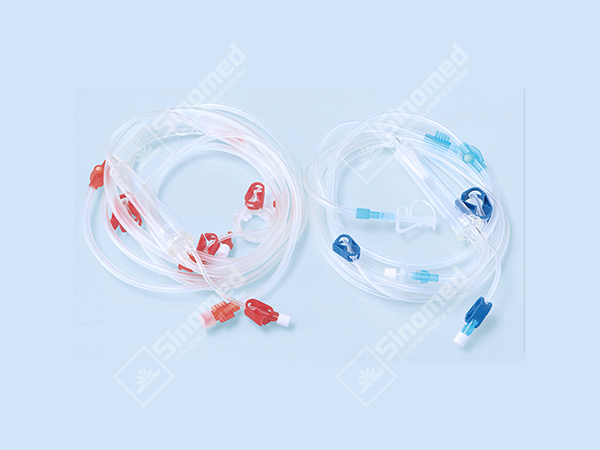
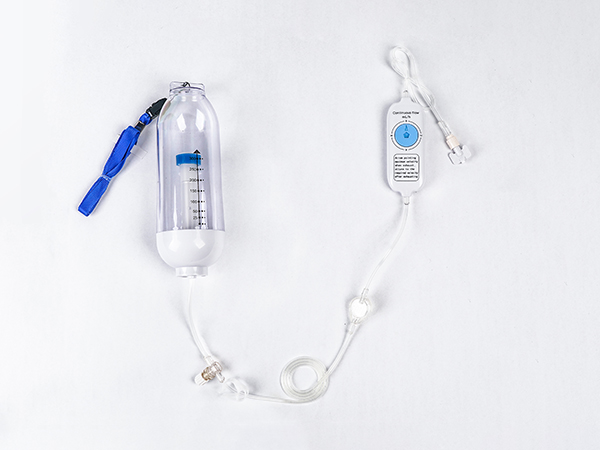
High-Quality Disposable Syringe Plant: Trusted Supplier for Your Needs
When it comes to high-quality disposable syringes, I understand the importance of finding a reliable supplier who can meet your specific needs. Our disposable syringe plant is dedicated to producing superior products that ensure safety and efficiency in every use. I pride myself on our rigorous quality control processes that guarantee each syringe meets stringent standards. With a focus on innovation and customer satisfaction, we strive to provide products that enhance your operations, whether in a clinical, research, or pharmaceutical setting. Collaborating with us means you’re choosing not just a product, but a partnership committed to quality and excellence. Let’s discuss how our disposable syringes can support your business goals and provide a seamless experience for your end-users. Quality, reliability, and customer service are at the heart of what we do, and I’m excited to be part of your success in the industry.
Disposable Syringe Plant Winning in 2025 From Concept to Delivery
As the demand for disposable syringes continues to rise globally, the journey from concept to delivery has never been more crucial for manufacturers. In 2025, companies will need to focus on innovative designs, efficient production processes, and robust supply chain management to remain competitive in a saturated market. Key advancements in materials science and automation technology will allow manufacturers to produce high-quality, cost-effective syringes that meet the stringent safety and regulatory standards expected by healthcare providers and patients alike. Moreover, sustainability and environmental considerations will play an increasing role in the industry. Manufacturers will need to embrace eco-friendly practices and materials to cater to the growing consumer demand for environmentally responsible products. Transparency in sourcing and production processes will not only enhance a company’s reputation but also build trust with global buyers looking for reliable partners in their procurement strategies. Collaboration with research institutions, healthcare professionals, and regulatory bodies will be fundamental in driving innovation and ensuring compliance. By focusing on these strategic areas, manufacturers can effectively position themselves for success in the disposable syringe market, meeting the needs of global procurement buyers who seek quality, reliability, and sustainability in their supply chains.
Disposable Syringe Plant Winning in 2025 From Concept to Delivery
| Stage | Description | Key Milestones | Projected Outcome |
|---|---|---|---|
| Concept Development | Initial design and prototype of the disposable syringe. | Prototype testing, user feedback collection. | Validated design specifications. |
| Regulatory Approval | Submission of design and safety data to regulatory bodies. | Approval from FDA/EMA. | Market entry authorization. |
| Production Planning | Setting up manufacturing processes and quality controls. | Installation of production lines. | Optimized production capacity. |
| Market Launch | Introduction of the disposable syringe to the market. | Launch campaigns, distribution network established. | Achieve target sales volume within the first year. |
| Feedback and Improvement | Collecting user feedback for continuous improvement. | Surveys, product reviews analysis. | Enhanced product features and user satisfaction. |
Related Products